COSMOSIL 1.8PBr
- Retain hydrophilic compounds with reversed‑phase chromatography
- Significantly improved separation compared to ODS columns
- High‑speed separation with UHPLC (1.8 μm particles)
Product Information
Material properties
| Packing Material | 1.8PBr | ||
|---|---|---|---|
| Silica Gel | Fully Porous Spherical High-Purity Silica | Surface Area (m2/g) | 340 |
| Particle Size (μm) | 1.8 | Bonded Phase | Pentabromobenzyl group |
| Pore Size (nm) | 12 | Bonding Type | Monomeric |
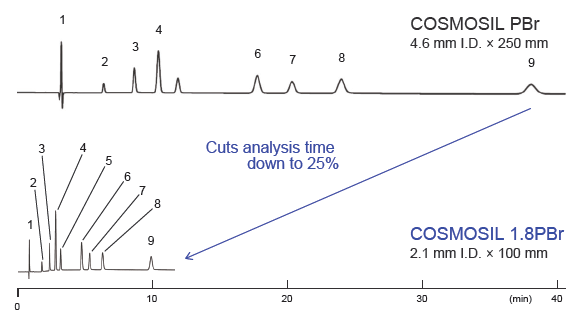
Rapid separation of hydrophilic compounds using UHPLC
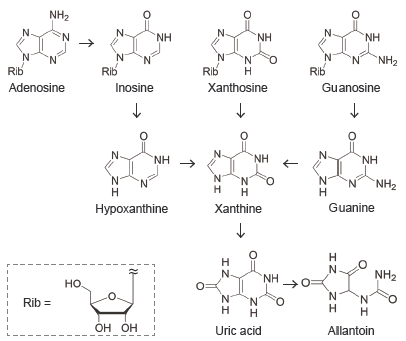
Rapid separation of purine metabolites
Using COSMOSIL PBr, the analysis of 9 purine nucleoside metabolites, including uric acid and allantoin, required approximately 40 minutes. Changing to COSMOSIL 1.8PBr, packed with smaller particles, allowed the analysis to be completed in approximately 10 minutes with equivalent separation.
| Conditions | |||
|---|---|---|---|
| Column size | 4.6 mm I.D. × 250 mm | Temperature | 30°C |
| Mobile phase | Methanol / 0.1% Formic acid aq. = 10 / 90 | Detection | UV 240 nm |
| Flow rate | PBr ; 1.0 mL/min, 1.8PBr ; 0.4 mL/min | Inj. Vol | 1.0 μL |
| Sample |
|  |
|
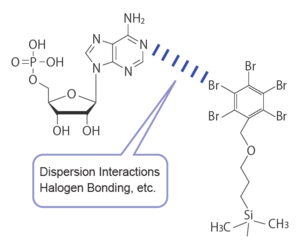
Hydrophilic compound retention by interaction with bromine
COSMOSIL PBr columns can be used in the same reversed‑phase mode as ODS columns; however, due to the high polarizability of bromine atoms, it exhibits unique retention and separation behavior. This is attributed to dispersion interactions and the recently recognized halogen bonding, which differ from conventional ODS columns. These interactions occur between highly polarizable atoms and π-electrons, as well as oxygen or nitrogen atoms possessing lone electron pairs. As a result, PBr columns can retain hydrophilic compounds that are difficult to retain on ODS columns, and enable the analysis of difficult compounds.
Imidazole dipeptide analysis
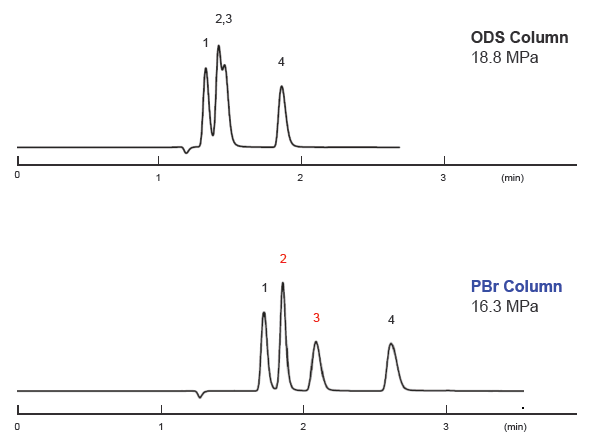
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 100 mm | Temperature | 40°C |
| Mobile phase | 20 mM Ammonium formate aq. | Detection | UV 220 nm |
| Flow rate | 0.2 mL/min | Inj. Vol | 0.5 μL |
| Sample |
| 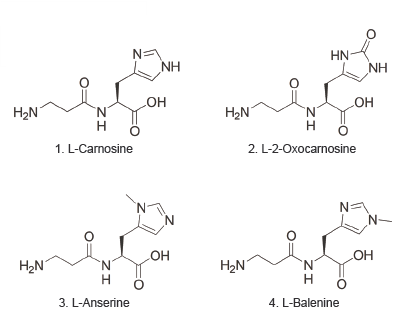 |
|
Nicotinamide metabolite analysis
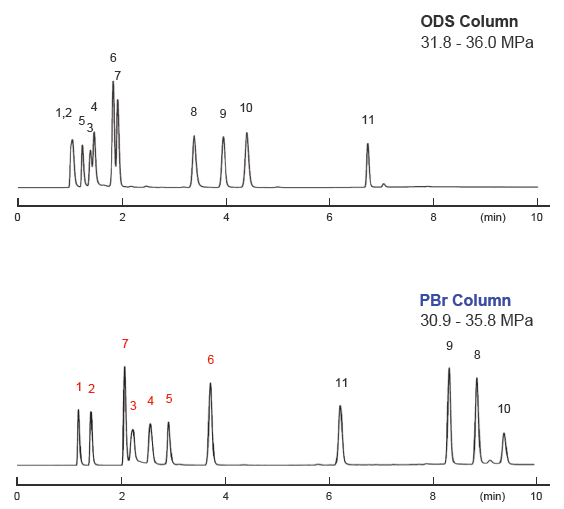
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 100 mm | Temperature | 30°C |
| Detection | UV 260 nm | Mobile phase | A ; Methanol / 20 mM Ammonium formate aq. = 5 / 95 B ; Methanol / 20 mM Ammonium formate aq. = 20 / 80 B conc. gradient ; 0% (0 → 3 min), 100% (3 → 6 min), 100% (6 → 10 min) |
| Flow rate | 0.3 mL/min | Inj. Vol | 1.0 μL |
| Sample |
|  |
|
Differences in separation characteristics due to stereoselectivity compared to ODS columns
With COSMOSIL PBr columns, selectivity derives not only from π-interactions of aromatic rings and dispersion interactions with bromine, but also structural configuration of the analytes. These effects can lead to improved separation on PBr columns, even when retention on ODS columns is sufficient. * Taniguchi A, et al. Chromatography. 2025;46:55.
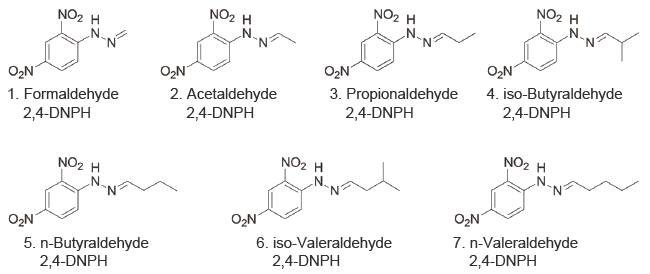
Malodorous aldehyde derivative analysis
In the analysis of odorous aldehyde derivatives regulated under Japan’s Offensive Odor Control Law, separation of certain isomers can be challenging. When using conventional reversed-phase columns, complex analytical conditions, such as a threecomponent mobile‑phase gradient, have been used. In contrast, analysis can be performed using 1.8PBr under simpler isocratic conditions using a two‑component mobile phase.
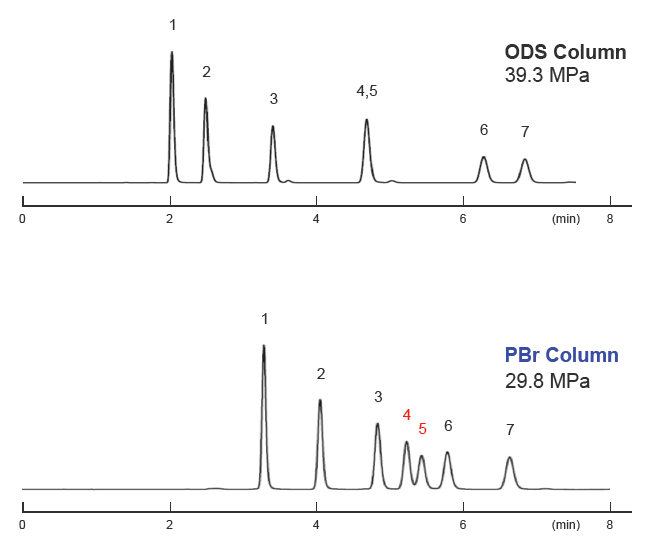
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 100 mm | Temperature | 30°C |
| Mobile phase | Methanol / Water = ** / ** ODS = 75 / 25 PBr = 90 / 10 | Detection | UV 360 nm |
| Flow rate | 0.3 mL/min | Inj. Vol | 0.4 μL |
| Sample |
|
||
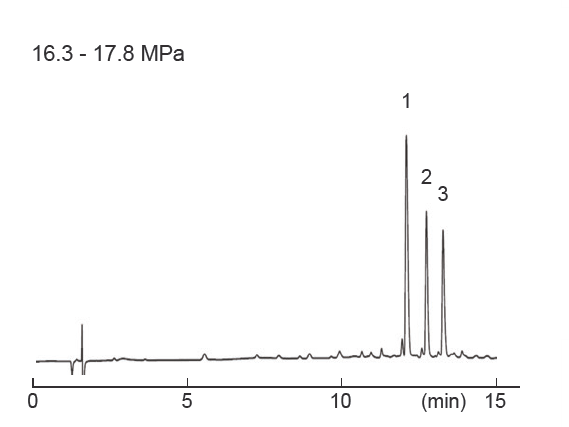
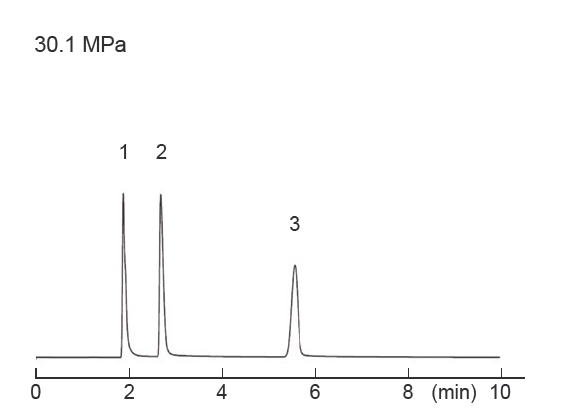
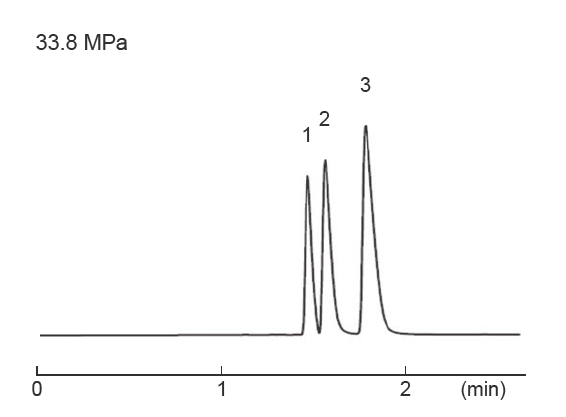
Differences in separation characteristics compared to PFP columns
PBr columns and pentafluorophenyl (PFP) columns both contain halogenated benzene. However, because PFP relies on dipole– dipole interactions, while PBr retains compounds primarily through dispersion interactions, their separation characteristics differ significantly. Both PFP and PBr columns showed improved separation compared to ODS columns, but the elution order was different. PFP exhibited strong retention of the fluorinated groups, -CF3 and ‑SF5, while PBr exhibited strong retention of the heavy halogen groups, -Br and ‑I, through dispersion interactions.
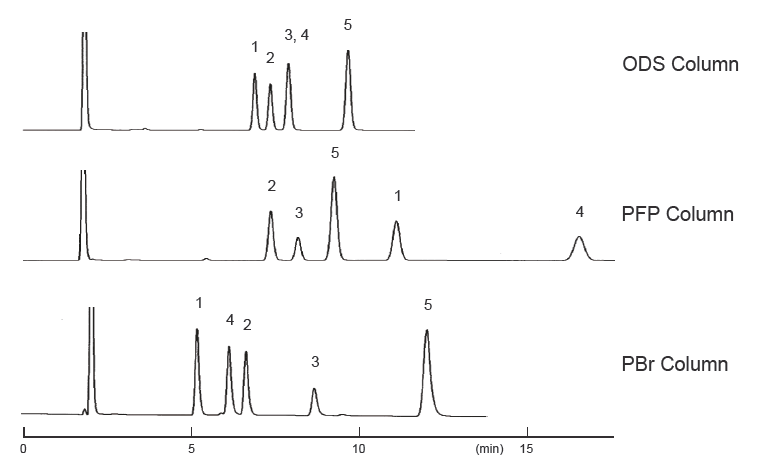
| Conditions | |||
|---|---|---|---|
| Column size | 4.6 mm I.D. × 150 mm | Sample |
 |
| Mobile phase | Methanol / Water = ** / ** ODS = 70 / 30 PFP = 60 / 40 PBr = 70 / 30 | ||
| Flow rate | 1.0 mL/min | ||
| Temperature | 30°C | ||
| Detection | UV 254 nm | ||
| Inj. Vol | 1.0 μL | ||
Applications
Purity of synthetic peptides
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 100 mm | Sample |
H-(D-Arg)n-NH2
|
| Mobile phase | A ; 0.1% TFA in water B ; 0.08% TFA in acetonitrile B conc. gradient ; 0 - 15% (0 - 15 min) | ||
| Flow rate | 0.2 mL/min | ||
| Temperature | 40°C | ||
| Detection | UV 220 nm | ||
Separation of adenosine phosphates
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 100 mm | Sample |
|
| Mobile phase | 20 mM Ammonium formate aq. | ||
| Flow rate | 0.4 mL/min | ||
| Temperature | 40°C | ||
| Detection | UV 260 nm | ||
Separation of isomers of highly hydrophilic compounds
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 150 mm | Sample |
|
| Mobile phase | 0.1% Formic acid aq. | ||
| Flow rate | 0.4 mL/min | ||
| Temperature | 40°C | ||
| Detection | UV 220 nm | ||
Separation of sugars
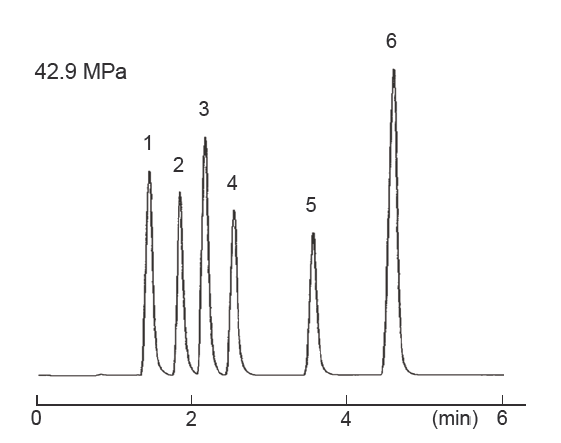
| Conditions | |||
|---|---|---|---|
| Column size | 2.1 mm I.D. × 150 mm | Sample |
|
| Mobile phase | Water | ||
| Flow rate | 0.4 mL/min | ||
| Temperature | 40°C | ||
| Detection | ELSD | ||
Note on Connector Type
Our UHPLC columns use the same connectors as Waters UPLC® (UHPLC) columns. This is different from our conventional COSMOSIL columns, which use the conventional Waters HPLC-compatible connectors. Attempting to connect an unsuitable fitting may result in it becoming stuck in the column.
Related Resources
For additional product information, please visit our website. You can access from the links below.
Ordering Information
COSMOSIL 1.8PBr Analytical Columns (Particle size : 1.8 µm)
COSMOSIL is a registered trademark of Nacalai Tesque, Inc. UPLC is a registered trademark of Waters Technologies Corporation.