Cell Culture Labware (EZSPHERE® for Spheroid Formation)

Three dimensional (3D) cell culture systems have gained in popularity as invaluable tools in broad applications of cell biology. 3D multi-cellular cell aggregates (spheroids) can be formed by using a low attachment culture surface. However, variability in forming spheroids has been a persistent problem. EZSPHERE is specifically designed to form a large number of uniformly sized spheroids and Embryoid Bodies (EBs).
Features
- The surface of EZSPHERE is coated with very low binding 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer.
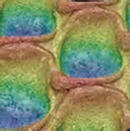
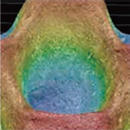
- EZSPHERE has a lot of evenly designed micro wells on the surface. Inoculated cells drop into the micro wells and form uniformly sized spheroids.
- Spheroids can be formed efficiently in the round shape wells.
Product Information
Features
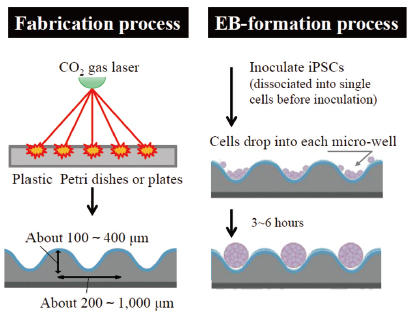
EZSPHERE is a unique micro-fabricated plastic vessel and very useful for mass-production of EBs with uniform size.
EZSPHERE Variety Pack (35 mm Dish)
 |
 |
 |
|
| Product No. | 4000-900SP | 4000-901SP | 4000-902SP |
| Diameter (µm) | approx. 500 | approx. 200 | approx. 500 |
| Depth (µm) | approx. 100 | approx. 100 | approx. 200 |
| No. of wells | approx. 2,700 | approx. 9,200 | approx. 2,700 |
 |
 |
 |
|
| Product No. | 4000-903SP | 4000-904SP | 4000-905SP |
| Diameter (µm) | approx. 800 | approx. 800 | approx. 1,400 |
| Depth (µm) | approx. 400 | approx. 300 | approx. 600 |
| No. of wells | approx. 1,300 | approx. 700 | approx. 260 |
Applications
EB formation, proliferation and differentiation protocols
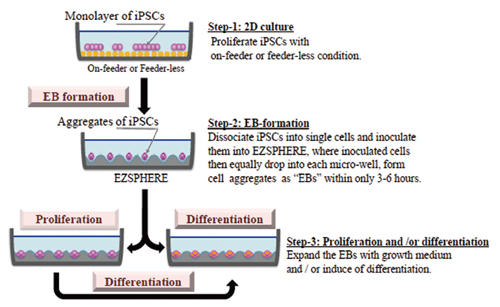
EB formation, proliferation and differentiation protocols
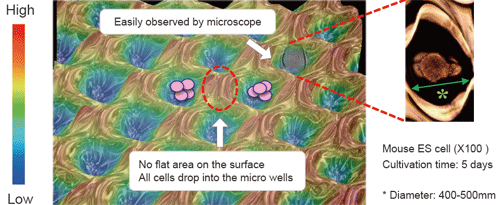
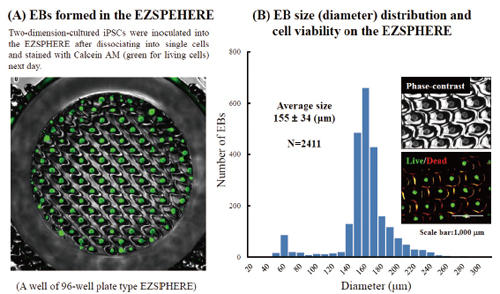
Fluorescence microscopy image of EBs obtained on the EZSPHERE (A). EBs created on the 35 mm dish type EZSPHERE were imaged and analyzed with the digital image analyzing software “Image J” to determine size distribution. Histogram of EB size (diameter) distribution. Fluorescence microscopy revealed that the almost EBs were alive with uniform size (B).
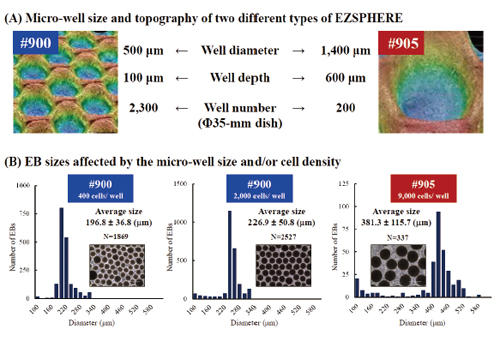
EB-size control with micro-well sizes or inoculating cell demsities
Two types of EZSPHERE #900 and #905 with micro-wells about 500 µm and 1,400 µm in diameter, respectively, were used to analysis the effect of the micro-well size or inoculated cell density on the EB sizes (A). Inoculation of iPSCs as 400 or 2,000 cells per micro-well on the same type of EZSPHERE #900 resulted in the formation of EBs with different sizes, while inoculating iPSCs as 9,000 cells per micro-well on the another EZSPHERE #905 resulted larger size of EBs (B). (Scale bars: 400 µm)
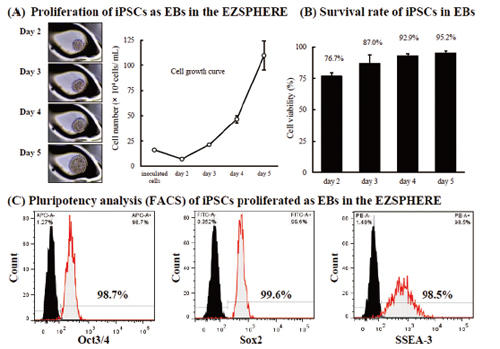
Proliferation of iPSCs with maintaining pluripotency
When iPSCs were cultured on EZSPHERE with feeder-free cell culture medium (mTeSR1), the formed EBs could proliferate at a good rate (A) with high viability (B). In the flow cytometry, these cells maintained high capacity of undifferentiated state (C).
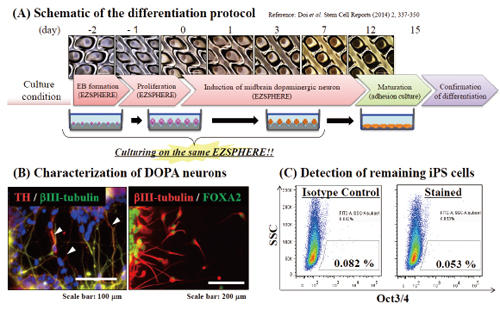
Induction of dopaminergic neurons from the EBs
Differentiation of EBs into dopaminergic neuron was attempted by using the EZSPHERE continuously throughout a series of steps from the EB-formation to induction of midbrain dopaminergic neuron (A). Immunofluorescence staining revealed differentiation of the EBs into midbrain neuron, which tyrosin hydroxylase (TH: white arrows) and FOXA2 positive (B). Flow cytometry analysis (FACS) with Oct3/4 antibody indicated that there was almost no iPSCs remained without differentiation (C).
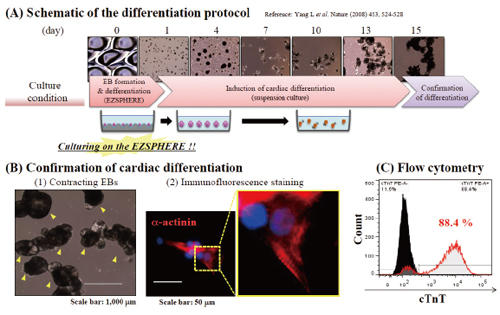
Induction of cardiomyocyte from the EBs
Differentiation of EBs, which prepared on the EZSPHERE, into cardiomyocytes was attempted (A). It was observed that most of EBs showed contracting (indicated with yellow arrow) at day 15 (B)-(1). EBs were dissociated and plated on gelatin coated slide, followed by α-actinin staining and sarcomere alignment (high magnification) was observed (B-2). Flow cytometry for cardiac troponin T (cTnT) positive cells revealed high differentiation efficiency (C).
References
- Hiroyuki Koike, Ran-Ran Zhang, Yasuharu Ueno, Keisuke Sekine, Yun-Wen Zheng, Takanori Takebe, Hideki Taniguchi Nutritional modulation of mouse and human liver bud growth through a branched-chain amino acid metabolism Development 2017 144: 1018-1024; doi: 10.1242/dev.143032
- Waseem K. Raja, Alison E. Mungenast, Yuan-Ta Lin, Tak Ko, Fatema Abdurrob, Jinsoo Seo, Li-Huei Tsai Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes PLOS ONE 11(9): e0161969. doi: 10.1371/journal.pone.0161969
- Ayako Aihara, Natsuki Abe, Koichiro Saruhashi, Tatsuro Kanaki, Taito Nishino Novel 3-D cell culture system for in vitro evaluation of anticancer drugs under anchorage-independent conditions Cancer Science Volume 107, Issue 12 December 2016 Pages 1858–1866 DOI: 10.1111/cas.13095
- Taku Nakayama, Shimpei Otsuka, Tatsuya Kobayashi, Hodaka Okajima, Kentaro Matsumoto, Yuichiro Hagiya, Keiji Inoue Taro Shuin, Motowo Nakajima, Tohru Tanaka, Shun-ichiro Ogura Dormant cancer cells accumulate high protoporphyrin IX levels and are sensitive to 5-aminolevulinic acid-based photodynamic therapy Scientific Reports 6, Article number: 36478 (2016) doi:10.1038/srep36478
- Ryohichi Sugimura, Deepak Kumar Jha, Areum Han, Clara Soria-Valles, Edroaldo Lummertz da Rocha, Yi-Fen Lu, Jeremy A. Goettel, Erik Serrao, R. Grant Rowe, Mohan Malleshaiah, Irene Wong, Patricia Sousa, Ted N. Zhu, Andrea Ditadi, Gordon Keller, Alan N. Engelman, Scott B. Snapper, Sergei Doulatov, George Q. Daley Haematopoietic stem and progenitor cells from human pluripotent stem cells Nature volume 545, pages 432–438 (25 May 2017) doi:10.1038/nature22370
Application references of AGC tissue culture products Sep. 2015 - Hiroki Sato, Alimjan Idiris, Tatsuaki Miwa, Hiromichi Kumagai
Microfabric Vessels for Embryoid Body Formation and Rapid Differentiation of Pluripotent Stem Cells
Scientific Reports 6, Article number: 31063 (2016) doi:10.1038/srep31063 - Matsuura K, Seta H, Haraguchi Y, Alsayegh K, Sekine H, Shimizu T, Hagiwara N, Yamazaki K, Okano T
TRPV-1-mediated elimination of residual iPS cells in bioengineered cardiac cell sheet tissues
Scientific Reports 6, Article number: 21747 (2016) doi:10.1038/srep21747 - Aikawa N, Suzuki Y, Takaba K
A Simple Protocol for the Myocardial Differentiation of Human iPS Cells
Biological and Pharmaceutical Bulletin Vol. 38 (2015) No. 7 Pages 1070-1075 - Shimada H, Hashimoto Y, Nakada A, Shigeno K, Nakamura T
Accelerated generation of human induced pluripotent stem cells with retroviral transduction and chemical inhibitors under physiological hypoxia
Biochemical and Biophysical Research Communications, Volume 417, Issue 2, Pages 659-664 - Zhang RR, Takebe T, Miyazaki L, Takayama M, Koike H, Kimura M, Enomura M, Zheng YW, Sekine K, Taniguchi H
Efficient Hepatic Differentiation of Human Induced Pluripotent
Stem Cells in a Three-Dimensional Microscale Culture Stem Cells and Tissue Repair Methods in Molecular Biology Volume 1210, 2014, pp 131-141
Downloads
Brochures
EZSPHERE® for Spheroid Formation ![]() (PDF 1.2MB)
(PDF 1.2MB)
Posters
Efficient Embryoid Body Formation from Human iPS Cells using Novel Microfabric Vessels (Poster from the 18th Takeda Science Foundation Symposium on Bioscience)
![]() (PDF 5.2MB)
(PDF 5.2MB)
An Innovative Method of Embryoid Body Formation Using Novel Microfabric Vessels
(Poster from World Conference on Regenerative Medicine, Leipzig 2015)
![]() (PDF 4MB)
(PDF 4MB)
Protocol
iPS spheroid formation in EZSPHERE devices
Ordering information
| Product Name | Well Size (µm) | No. of Wells | Product No. | Qty. |
|---|---|---|---|---|
| EZSPHERE SP Dish 35 mm | Diameter: 400-500 Depth: 100-200 |
2,700/dish | 4000-900SP | 10 |
| EZSPHERE SP Dish 60 mm | 6,500/dish | 4010-900SP | 10 | |
| EZSPHERE SP Dish 100 mm | 17,000/dish | 4020-900SP | 10 | |
| EZSPHERE SP 6-Well Plate | 2,700/well* | 4810-900SP | 5 | |
| EZSPHERE SP 96-Well Plate | 100/well* | 4860-900SP | 5 | |
| EZSPHERE SP Dish 35 mm Type 902 | 2,700/dish | 4000-902SP | 10 | |
| EZSPHERE SP Dish 35 mm Type 903 | Diameter: 800 Depth: 400 |
1,300/dish | 4000-903SP | 10 |
| EZSPHERE SP Dish 35 mm Type 904 | Diameter: 800 Depth: 300 |
700/dish | 4000-904SP | 10 |
| EZSPHERE Variety Pack (35 mm Dish) | 6 PC | 4000-9VP | 1 set |
*Number of spheroid wells per large well