COSMOSIL Index
COSMOSIL Ultra-High Performance Columns
Specifications
| Packing Material | 2.5 C18-MS-II |
2.5 Cholester |
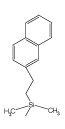
2.5 πNAP |
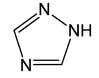
2.5 HILIC |
|---|---|---|---|---|
| USP Code | L1 | L101 | L104 | |
| Silica Gel | High Purity Porous Spherical Silica | |||
| Average Particle Size | 2.5 µm | |||
| Average Pore Size | approx. 130 Å | |||
| Specific Surface Area | approx. 330 m2/g | |||
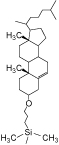
| Stationary Phase | 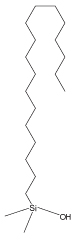 |
 |
 |
 |
| Octadecyl Group | Cholesteryl Group | Naphtylethyl Group |
Triazole | |
| Bonding Type | Monomeric | - | ||
| Main Interaction | Hydrophobic Interaction | Hydrophobic Interaction Molecular Shape Selectivity |
Hydrophobic Interaction π-π Interaction |
Hydrophilic Interaction, Anion Exchange |
| End Capping Treatment | Near-perfect Treatment | - | ||
| Features | - Multi-purpose C18 column - Suitable for basic compounds. |
- Usable under the same condition as C18. -Strong molecular shape selectivity |
- Stronger π-π interaction than Phenyl columns. | -Suitable for non-retaining by C18 |
COSMOSIL 2.5C18-MS-II
- Monomeric C18 phase for multi-purpose separations
- Low back pressure
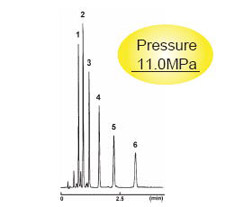
Pressure Comparison with Competitor's 2 µm Columns
3.0 mm I.D . x 75 mm
| 2.5C18-MS-II | Competitor's 2 µm C18 |
|---|---|
 |
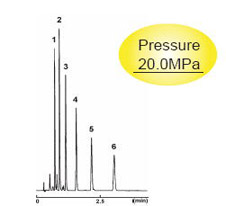 |
| Condition | |||
|---|---|---|---|
| Column Size | 3.0 mm I.D . x 75 mm | Sample |
|
| Mobile Phase | Acetonitrile : H2O = 70 : 30 | ||
| Flow Rate | 1 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 254 nm | Injection Vol. | 1.0 µl |
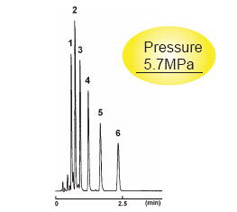
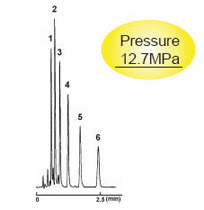
2.0 mm I.D . x 50 mm
| 2.5C18-MS-II | Competitor's 2 μm C18 |
|---|---|
 |
 |
| Condition | |||
|---|---|---|---|
| Column Size | 2.0 mm I.D . x 50 mm | Sample |
|
| Mobile Phase | Acetonitrile : H2O = 70 : 30 | ||
| Flow Rate | 0.4 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 254 nm | Injection Vol. | 0.5 µl |
Brochures
- COSMOSIL Ultra-High Performance Column
 (1.2 MB)
(1.2 MB)
Ordering Information
COSMOSIL 2.5C18-MS-II
COSMOSIL C18-MS-II (Particle size : 3 ・ 5 ・15 µm) is also available.
COSMOSIL 2.5Cholester
- Unique rigid Cholesteryl structure
- Usable under the same condition as ODS
- 2.5 µm silica packing material
Improved Separation
COSMOSIL 2.5Cholester offers improved resolution for compounds difficult to analyze with C18 without changing analytical condition.
Separation of Catechins
| 2.5Cholester | Competitor's 2 µm C18 |
|---|---|
.jpg) |
.jpg) |
| Condition | |||
|---|---|---|---|
| Column Size | 3.0 mm I.D. x 75 mm | Sample |
|
| Mobile Phase | A: Acetonitrile : 20 mmol/l Phosphate Buffer (pH2.5)= 10 : 90 B: Acetonitrile : 20 mmol/l Phosphate Buffer (pH2.5)= 30 : 70 B: 0→ 100%/ 5 min Linear Gradient |
||
| Mixer | 0.5 ml | ||
| Flow Rate | 1 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 280 nm | Injection Vol. | 1.0 µl |
Separation of Saikosaponins
| 2.5Cholester (2.0 mm I.D. x 50 mm) | Competitor's 1.7 µm C18(2.1 mm I.D. x 50 mm) |
|---|---|
.jpg) |
.jpg) |
| Condition | |||
|---|---|---|---|
| Mobile Phase | Acetonitrile: 0.05%NaH2PO4 aq. = 30 : 50 | Sample |
|
| Flow Rate | 0.7 ml/min | ||
| Temperature | 50 °C | ||
| Detection | UV206 nm | Injection Vol. | 1.0 µl |
Separation of Geometrical Isomers
| 2.5Cholester | Competitor's 2 μm C18 |
|---|---|
.jpg) |
.jpg) |
| Condition | |||
|---|---|---|---|
| Column Size | 3.0 mm I.D. x 75 mm | Sample |
|
| Mobile Phase | Acetonitrile : Water = 70 : 30 | ||
| Flow Rate | 1.0 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 254 nm | Injection Vol. | 1.0 µl |
Comparison of Planarity Selectivity
Cholester shows greater planarity selectivity. (Planarity : o-Terphenyl < Triphenylene)
| 2.5Cholester | Competitor's 2 µm C18 |
|---|---|
.jpg) |
.jpg) |
| Condition | |||
|---|---|---|---|
| Column Size | 3.0 mm I.D. x 75 mm | Sample |
 |
| Mobile Phase | Methanol : Water = 90 : 10 | ||
| Flow Rate | 1 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 254 nm | ||
Brochures
- COSMOSIL Ultra-High Performance Column
 (1.2 MB)
(1.2 MB)
- COSMOSIL/COSMOCORE Cholester Series Application Notebook and Reference
Lis
 (14 MB)
(14 MB)
Poster at HPLC2009
ADVANTAGES OF A NOVEL STATIONARY PHASE USING 2.5 µM PARTICLES FOR
ULTRA-FAST LIQUID CHROMATOGRAPHY
Analysis of Natural Compounds by 2.5Cholester
Ordering Information
COSMOSIL 2.5Cholester
COSMOSIL Cholester (Particle size : 5 µm) is also available.
COSMOSIL 2.5πNAP
- Low back pressure (2.5 µm silica gel)
- Naphthalene bonded stationary phase
- Stronger π-π interactions than Phenyl columns
Improved Separation
COSMOSIL 2.5πNAP provides greater performance in separating positional isomers and other closely related compounds which are difficult to analyze with C18.
Positional Isomers of Tolunitriles
| 2.5πNAP | Competitor's 2 µm C18 |
|---|---|
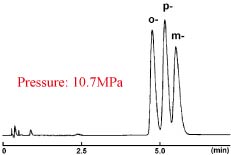 |
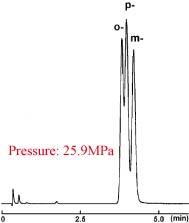 |
| Condition | |||
|---|---|---|---|
| Column Size | 2.0 mm I.D. x 50 mm | Sample |
 |
| Mobile Phase | Methanol / Water = 35 : 65 |
||
| Flow Rate | 0.4 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 254 nm | ||
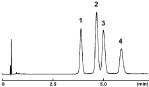
Positional Isomers of Tocopherols
| 2.5πNAP | Competitor's 2 µm C18 |
|---|---|
 |
 |
| Condition | |||
|---|---|---|---|
| Column Size | 3.0 mm I.D. x 75 mm | Sample |
|
| Mobile Phase | 2.5πNAP: Methanol / Water = 85 : 15 Competitor's 2µm C18: Methanol / Water = 95 : 5 |
||
| Flow Rate | 1.0 ml/min | ||
| Temperature | 40 °C | Detection | UV 295 nm |
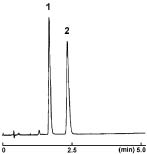
Berberine
| 2.5πNAP | 2.5C18-MS-Ⅱ |
|---|---|
 |
 |
| Condition | |||
|---|---|---|---|
| Column Size | 3.0 mm I.D. x 75 mm | Sample |
 |
| Mobile Phase |
Methanol/ 20mmol/l Phosphate Buffer(pH2.5)
|
||
| Flow Rate | 1.0 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 254 nm | ||
Ordering Information
COSMOSIL 2.5πNAP
COSMOSIL πNAP Packed Column (Particle size :5 µm) is also available.
COSMOSIL 2.5HILIC
- Ultra-High Performance using 2.5 µm particles
- Triazole bonded stationary phase
- Alternative selectivity to other HILIC columns
Ultra-High-Speed Analysis (Oxidation marker analysis)
COSMOSIL 2.5HILIC can be used with any conventional LC systems.
| HILIC (5 µm) (4.6 mm I.D. - 250 mm) |
2.5HILIC (2.5 µm) (3.0 mm I.D. - 100 mm) |
|---|---|
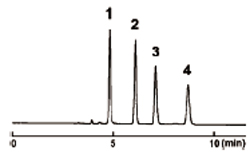 |
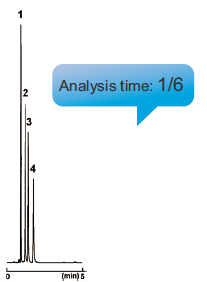 |
| Condition | |||
|---|---|---|---|
| Mobile Phase |
Acetonitrile/ 10mmol/l Ammonium Acetate = 80/20 |
Sample |
|
| Flow Rate | 1.0 ml/min | ||
| Temperature | 40 °C | ||
| Detection | UV 249 nm | Inj. Vol | 1.0 µl |
Applications
| Umami Components |
|---|
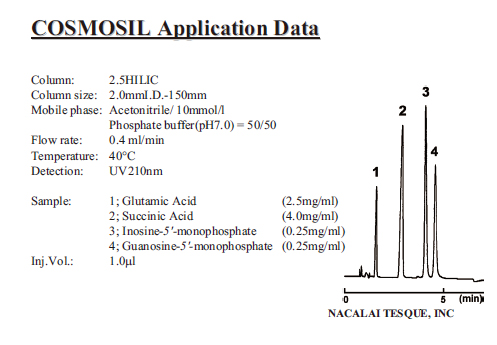 |
| Adenine Nucleotide |
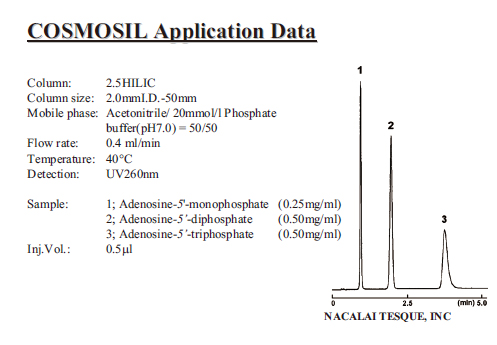 |
| Taurine/Hypotaurine |
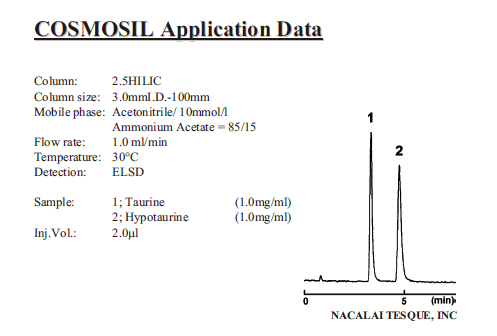 |
| Haloacetic Acid |
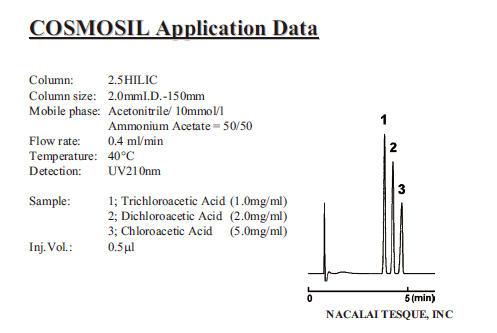 |
Brochures
- COSMOSIL Ultra-High Performance Column
 (1.2 MB)
(1.2 MB)
Ordering Information
COSMOSIL 2.5HILIC
COSMOSIL HILIC Packed Column (Particle size :5 µm) is also available.
Usage information
| Column | 2.5 µm MS-II, Cholester | |
|---|---|---|
| I.D. (mm) | 2.0 | 3.0 |
| Washing method | 1. Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. For example, if your mobile phase was 50:50 methanol/20 mmol/l phosphate buffer, wash with 50:50 methanol/water. 2. (If you used a buffer, always do step 1 first!) Remove adsorbed compounds and fix unstable baselines by washing with methanol and/or THF. |
|
| Storage conditions | Short-term (up to a week): Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. For example, if your mobile phase was 50:50 methanol/20 mmol/l phosphate buffer, wash with 50:50 methanol/water. Long-term: Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. Then, replace the solvent with 70% methanol or acetonitrile : 30% water. In either case, tightly plug the column, and store in a cool and dry place. |
|
| Recommended flow rate | 0.4 ml/min | 1 ml/min |
| Usable pH range | pH2~pH7.5 | |
| Max. pressure | 30MPa | |
| Temperature range | The maximum usable temperature is 60℃. However. for regular use, please use at a constant temperature between 20°C and 50°C. | |
| Usable solvents | Any solvent that will not dissolve the silica gel (such as alkaline solutions) or cleave the stationary phase (such as very acidic solutions) is usable. Note: For solvents with high viscosity, please watch the system pressure and keep it below 30 MPa. Also, please wash the column after using acidic mobile phases or solvents with high freezing points. |
|
| Other instructions | - Buffer concentration is usually sufficient at 0.005 - 0.02 mol/L. - Always filter mobile phases using a 0.45 µm or finer filter before use. - Reproducibility may worsen when using a mobile phase that is more than 90% water. |
|
| Column | 2.5 µm πNAP | |
|---|---|---|
| I.D. (mm) | 2.0 | 3.0 |
| Washing method | 1. Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. For example, if your mobile phase was 50:50 methanol/20 mmol/l phosphate buffer, wash with 50:50 methanol/water. 2. (If you used a buffer, always do step 1 first!) Remove adsorbed compounds and fix unstable baselines by washing with methanol and/or THF. |
|
| Storage conditions | Short-term (up to a week): Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. For example, if your mobile phase was 50:50 methanol/20 mmol/l phosphate buffer, wash with 50:50 methanol/water. Long-term: Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. Then, replace the solvent with 70% methanol or acetonitrile : 30% water. In either case, tightly plug the column, and store in a cool and dry place. |
|
| Recommended flow rate | 0.4 ml/min | 1 ml/min |
| Usable pH range | pH2~pH7.5 | |
| Max. pressure | 30MPa | |
| Temperature range | The maximum usable temperature is 60°C. However. for regular use, please use at a constant temperature between 20°C and 50°C. | |
| Usable solvents | Any solvent that will not dissolve the silica gel (such as alkaline solutions) or cleave the stationary phase (such as very acidic solutions) is usable. Note: For solvents with high viscosity, please watch the system pressure and keep it below 30 MPa. Also, please wash the column after using acidic mobile phases or solvents with high freezing points. |
|
| Other instructions | - Buffer concentration is usually sufficient at 0.005 - 0.02 mol/L. - Always filter mobile phases using a 0.45 µm or finer filter before use. - Acetonitrile is not recommended as a mobile phase. |
|
| Column | 2.5 µm HILIC | |
|---|---|---|
| I.D. (mm) | 2.0 | 3.0 |
| Washing method | 1. Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. For example, if your mobile phase was 50:50 acetonitrile/20 mmol/l phosphate buffer, wash with 50:50 acetonitrile/water. 2. Remove adsorbed compounds and fix unstable baselines: Wash with 50:50 acetonitrile/water or up to 100% water. |
|
| Storage conditions | Remove buffer, salts and/or acid from column: wash for 10-15 min. using the mobile phase last used, without buffer, salts or acid. Then, replace the solvent with 90:10 acetonitrile/water. Tightly plug the column, and store in a cool and dry place. |
|
| Recommended flow rate | 0.4 ml/min | 1 ml/min |
| Usable pH range | pH2~pH7.5 | |
| Max. pressure | 30MPa | |
| Temperature range | The maximum usable temperature is 60°C. However. for regular use, please use at a constant temperature between 20°C and 50°C. | |
| Usable solvents | - Acetonitrile/water mobile phases are recommended. | |
| Other instructions | - Retention increases with increased acetonitrile concentration. - Acetonitrile concentration should be within 0-95% (usually 50-95%). - Retention will decrease when using methanol/water mobile phases. - Only use HPLC-grade solvents. - It is necessary to use salts or buffers for dissociating compounds. However, these have low solubility in the high-organic mobile phases used in HILIC mode. Phosphate buffers, widely used in reversed-phase, are not suitable for HILIC for this reason. When using salts or buffers, the acetonitrile concentration should be 70% or less. Before use, please confirm that the additives are completely soluble in the mobile phase. |
|